Journal of Cancer Research & Therapy
An International Peer-Reviewed Open Access Journal
ISSN 2052-4994


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Cancer Research & Therapy
Volume 5, Issue 9, October 2017, Pages 56–60
Case reportOpen Access
The first American cancer patient to receive dicycloplatin chemotherapy: A case report
-
Jing Jie Yu1,*
 ,
Mohamad W. Salkini2,
Shunchang Jiao5,
Thomas Hogan3,
Yi Guo1,
Xiaobing Liang1,
Bo Yang5,
Li Zhang5 and
Kymberly Gyure4
,
Mohamad W. Salkini2,
Shunchang Jiao5,
Thomas Hogan3,
Yi Guo1,
Xiaobing Liang1,
Bo Yang5,
Li Zhang5 and
Kymberly Gyure4
- 1 West Virginia University Cancer Institute, School of Medicine and School of Pharmacy, West Virginia University, Morgantown, WV, USA
- 2 Department of Surgery/Urology, School of Medicine, West Virginia University, Morgantown, WV, USA
- 3 Department of Medicine, School of Medicine, West Virginia University, Morgantown, WV, USA
- 4 Department of Pathology, School of Medicine, West Virginia University, Morgantown, WV, USA
- 5 Oncology Department of Internal Medicine, PLA Hospital, Beijing, China
*Corresponding author: Jing Jie Yu, MD, WVU Cancer Institute, School of Medicine and School of Pharmacy, West Virginia University, Morgantown, WV, USA. Tel.: 304-293-8661; E-mail: jyu@hsc.wvu.edu
Received 15 July 2017 Revised 29 September 2017 Accepted 4 October 2017 Published 10 October 2017
DOI: http://dx.doi.org/10.14312/2052-4994.2017-11
Copyright: © 2017 Yu JJ, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Dicycloplatin (DCP), an effective platinum drug, was developed in China and approved by Chinese FDA in 2012. Its side effects are more bearable than those of cisplatin and carboplatin. The bladder cancer patient presented here was a 65-year-old Caucasian American male diagnosed at West Virginia University (WVU) Hospital in the USA and received 8 weeks of dicycloplatin chemotherapy in Beijing in 2016. He experienced moderate fatigue and aches but no vomiting or hair loss. His blood values decreased but remained in normal range. Quarterly cystoscopic follow-ups at WVU Hospital found no tumor recurrence. Studies of dicycloplatin-induced activation of the molecular signaling pathway in response to DNA damage, including investigation of Chk2, BRCA1 and p53, demonstrated that the signaling pathway is activated only in cancer cells, not in normal cells. Other in vitro data also indicate DCP mainly affects tumor cells, largely bypassing normal cells. These findings may explain DCP's more bearable side effects. Dicycloplatin may offer chemotherapeutic advantages over some platinum drugs in cancer treatment.
Keywords: dicycloplatin; chemotherapy; bearable side effects; bladder cancer; molecular mechanism pathway
IntroductionTop
Cancer is one of the leading causes of morbidity and mortality worldwide, with approximately 14.1 million new cases in 2012; of these 7.4 million cases were in men and 6.7 million in women [1]. The annual number of new cases is expected to increase to 24 million by 2035 [2].
The rate of cancer death in the United States for men and women combined fell 25% from its peak in 1991 to 2014. This decline translates to the prevention of millions of cancer deaths [3]. That is significant progress; however, 1,685,210 new cancer cases and 595,690 cancer deaths are projected in the USA in 2016 [3].
Platinum-containing antineoplastic agents remain a cornerstone of chemotherapy for many cancers, including bladder cancer. The primary platinum drugs are cisplatin and carboplatin. Unfortunately, the side effects of these drugs are often severe, causing some patients to stop treatment. Toxic effects include myelosuppression, nephrotoxicity, hepatotoxicity, neurotoxicity, ototoxicity, and nausea and vomiting. These are often constraints to giving full dosage and to long-term use.
The need for highly-potent platinum anticancer drugs with low toxicity and broad-spectrum effectiveness led to the development of dicycloplatin (DCP). DCP is a non-covalent association of complexes or molecules (a supramolecule) assembled through four strategically-placed hydrogen bonds. Clinical observations show that DCP possesses superior anticancer efficacy through its broad-spectrum effects, high antineoplastic activity, low toxicity, and good tissue penetration [4-6].
DCP for intravesical injection was developed for a Chinese phase I human clinical trial completed in 2006. From 2007 to 2009 a double-blind, randomized multicenter phase II clinical trial comparing dicycloplatin and paclitaxel to carboplatin and paclitaxel in non-small cell lung cancer was completed (NSCLC) [7, 8]. Two hundred and forty chemotherapy-naïve patients with advanced NSCLC were recruited from 15 multi-center/hospitals in China. The study concluded that DCP was not inferior to carboplatin in efficacy or toxicity. In March 2012, dicycloplatin injection was approved for chemotherapy by the Chinese FDA [4-6].
Bladder cancer (BC) caused 170,000 deaths annually worldwide. It is the 4th most common cancer in men and the 9th in women in the United States. More than 50,000 men and 16,000 women are diagnosed with BC each year in the US [9, 10]. Bladder cancer arises from the epithelial lining of the urinary bladder. The most common type is transitional cell or urothelial cell carcinoma, which can also arise in the histologically similar urothelium of the renal pelvis or ureters.
BC commonly presents with hematuria -- visible (macroscopic) or microscopic -- noted in 80-90% of patients. It may also present with painful urination, increased frequency of urination, or urgency. Advanced BC may be accompanied by pelvic, flank, or bone pain, leg edema or a palpable pelvic mass on physical examination. Tobacco smoking is associated with over half of the cases in men and one-third in women [11, 12], with an almost linear relationship between smoking duration (in years), pack per years, and BC risk. Although quitting smoking lowers the risk, former smokers are likely always at a higher risk of BC versus never smokers [13].
The standard diagnosis for bladder cancer is cystoscopic biopsy. Transurethral resection of bladder tumor (TURBT) during cystoscopy and bimanual examination done pre- and post-TURBT can help assess whether the tumor is fixed ("tethered") to the pelvic wall. The pathological classification obtained at TURBT is of fundamental importance in choosing treatment and/or follow-up routines [14].
BC treatment depends on depth of tumor invasion. Non-muscle invasive tumors can be removed by transurethral resection of bladder tumor and serve primarily for pathological staging [14]. Intravesical immunotherapy with Bacillus Calmette–Guérin (BCG) is typically used to treat and to prevent recurrence of non-muscle invasive tumors [15] and in randomized trials is superior to standard chemotherapy [16]. However, the rate of recurrence after adjuvant BCG treatment in bladder cancer is about 50% (90% without BCG). Untreated, non-muscle invasive tumors may gradually infiltrate the muscular wall of the bladder, requiring cystectomy and urinary diversion into an isolated bowel loop (an ileal conduit or urostomy) or a substitute bladder (neobladder) from a segment of intestinal tissue. For muscle invasive BC, the gold standard is cystectomy (partial or radical cystectomy depending on tumor number and size). Micrometastatic dissemination is now treated with “neoadjuvant” or anterior chemotherapy for three or four cycles, than proceeding to major surgery [14-16].
Approximately 70% of new urothelial bladder cancer cases are classified as non-muscle invasive [17]. The initial treatment of presumed non-muscle invasive bladder tumors is a complete transurethral resection of all visible bladder tumor with adequate depth to include the muscularis propria [18]. To prevent recurrence of non-muscle invasive tumors (T1, Ta), resection is typically followed by intravesical treatment if the tumor was high-grade urothelial carcinoma or in the event of large tumors or multifocal disease. BCG is the most commonly used agent for intravesical therapy. It is the standard protocol. Other intravesical agents have been compared with BCG but most are inferior and none consistently proven to be superior [19-23].
Case reportTop
A 65-year-old Caucasian American male with increasing hematuria over a 4-month period, without lower urinary tract symptoms, was seen in June of 2016 at West Virginia University (WVU) Hospital, Morgantown, WV, USA. The patient was a former smoker. The work up, with CT-Urography and cystoscopy, showed a 1.5 cm bladder mass. Transurethral resection was performed on June 30, 2016. The pathologic diagnosis was non-invasive high-grade papillary urothelial carcinoma involving the right lateral wall bladder tumor (Ta) (Figure 1). Immunotherapy with BCG and surveillance cystoscopy were recommended to the patient. The patient declined BCG treatment course but elected cystoscopic surveillance every three months at WVU after dicycloplatin chemotherapy in Beijing, China.

Of note, the patient is a former smoker. His mother died of ovarian cancer at age 50 and his father had advanced prostate cancer at the time of his death at age 81 from other causes. The patient’s family history and his familiarity with DCP through his work as a medical writer led him to elect DCP. In July 2016, the patient traveled to Beijing where he received eight weekly DCP IV infusions.
The patient was evaluated by a Chinese oncologist at People’s Liberation Army General Hospital (also known as Beijing 301 Hospital); consent forms were signed. Baseline blood counts and chemistry were evaluated prior to chemotherapy, then weekly before each treatment.The DCP treatment protocol at Beijing 301 Hospital began with omeprazole and reduced glutathione sodium dissolved separately in 100 ml of 0.9% saline solution and infused, their purpose according to medical staff to protect the liver and kidneys. An intramuscular injection of diphenhydramine was given before each treatment. Dicycloplatin 300 mg was dissolved in 250 ml 5% glucose solution and infused over one hour. The patient was treated in the Day Ward of the Tumor Building at Beijing 301 Hospital and observed for 30 min afterwards.
The patient received one cycle of 300 mg of DCP by IV weekly for eight weeks. In addition, An-Tuo-Ke-Jin, the capsule form of dicycloplatin, was administered orally to achieve the standard weekly DCP dosage. One capsule contains 3 mg of dicycloplatin; four capsules were taken three times a day for one and half months.
Adverse effects included mild nausea, moderate fatigue, and, during the last weeks, back and leg aches. There was no vomiting or hair loss. Weekly blood counts and chemistry results are shown in Tables 1 and 2. They remained within normal limits, though there were notable decreases in red blood cells, white blood cells, and platelets.
| CBC with differential | Reference range | Unit | pre-DCP | DCP treatment | ||||||
| 7/20/2016 | 7/26/2016 | 08-02-2016 | 08-09-2016 | 8/16/2016 | 8/23/2016 | 8/30/2016 | 09-06-2016 | |||
| Hemoglobin | 137~179(M); 116~155(F) | g/L | 137 | 142 | 137 | 134 | 132 | 139 | 135 | 134 |
| RBC | 4.3-5.9(M); 3.9~5.2(F) | 10˄12/L | 4.63 | 4.81 | 4.64 | 4.51 | 4.49 | 4.31 | 4.42 | 4.34 |
| WBC | 3.5~10 | 10˄9/L | 7.37 | 7.04 | 6.32 | 5.32 | 4.7 | 4.62 | 3.9 | 4.04 |
| Neutrophils | 0.50~0.70 | 10˄9/L | 0.553 | 0.603 | 0.591 | 0.624 | 0.48 | 0.591 | 0.507 | 0.488 |
| Lymphocytes | 0.20~0.40 | 10˄9/L | 0.328 | 0.295 | 0.32 | 0.258 | 0.294 | 0.303 | 0.408 | 0.408 |
| Monocytes | 0.03~0.08 | 10˄9/L | 0.081 | 0.071 | 0.059 | 0.075 | 0.083 | 0.076 | 0.059 | 0.077 |
| Basophils | 0.01~0.05 | 10˄9/L | 0.031 | 0.024 | 0.024 | 0.041 | 0.132 | 0.091 | 0.018 | 0.02 |
| Eosinophils | 0.00~0.01 | 10˄9/L | 0.007 | 0.007 | 0.006 | 0.002 | 0.011 | 0.011 | 0.008 | 0.007 |
| PCV | 0.4~0.52(M); 0.37~0.47(F) | L/L | 0.413 | 0.429 | 0.419 | 0.419 | 0.401 | 0.421 | 0.407 | 0.407 |
| MCV | 80~100 | fl | 89.2 | 89.2 | 90.3 | 92.9 | 89.3 | 91.3 | 92.1 | 93.8 |
| MCH | 27~34 | pg | 29.6 | 29.5 | 29.5 | 29.7 | 29.4 | 30.2 | 30.5 | 30.9 |
| MCHC | 320~360 | g/L | 332 | 331 | 327 | 320 | 329 | 330 | 332 | 329 |
| RDW | <14.5 | % | 13.10% | 12.9 | 13.8 | 13.6 | 14.1 | 14.6 | 14.9 | 14.8 |
| Platelets | 100~300 | 10˄9/L | 248 | 267 | 244 | 225 | 244 | 187 | 147 | 177 |
| MPV | 6.8~12.8 | fl | 12.2 | 12 | 11.7 | 11.3 | 11.3 | 11.2 | 11.4 | 10.5 |
| Basic metabolic panel | Reference | Unit | Pre-DCP | DCP treatment | ||||||
| 7/21/2016 | 7/26/2016 | 8/2/2016 | 08/09/2016 | 8/16/2016 | 8/23/2016 | 8/30/2016 | 09/06/2016 | |||
| Potassium | 3.5 - 5.5 | mmol/L | 4.44 | 5.03 | 4.8 | 5.14 | 4.7 | 5.05 | 5.21 | 5.1 |
| Sodium | 130 - 150 | mmol/L | 138.5 | 138.7 | 133.7 | 139.7 | 138.9 | 141.3 | 134.9 | 138.6 |
| Chloride | 94 - 110 | mmol/L | 94.9 | 95.6 | 97.7 | 99.2 | 99.6 | 98.9 | 101.3 | 100.3 |
| Calcium | 2.09 - 2.54 | mmol/L | 2.36 | 2.37 | 2.33 | 2.3 | 2.28 | 2.35 | 2.33 | 2.33 |
| Albumin | 35 - 50 | g/L | 42.9 | 46.8 | 44.3 | 42.4 | 42.5 | 43.7 | 42.2 | 40.8 |
| Total bilirubin | 0 - 21 | mmol/L | 5.1 | 8.2 | 9.1 | 8.2 | 8.6 | 10.8 | 12.1 | 9.6 |
| Alkaline phosphatase | 0 - 130 | U/L | 101.3 | 107.4 | 94.4 | 100.7 | 113.7 | 100.9 | 91.9 | 94.2 |
| Creatinine | 30 - 110 | mmol/L | 68.8 | 71.8 | 78 | 69.6 | 68.7 | 69.8 | 71.1 | 75 |
| Urea | 1.8 - 7.5 | mmol/L | 3.45 | 3.21 | 3.38 | 3.74 | 3.45 | 3.37 | 3.32 | 4.21 |
| Uric acid | 104 - 444 | mmol/L | 265.6 | 329 | 337.2 | 300.6 | 347.5 | 343.4 | 391 | |
| ALT | 0 - 40 | U/L | 41.4 (H) | 45.8 (H) | 46.7 (H) | 44.1 (H) | 42.0 (H) | 61.8 (H) | 48.7 (H) | 47.1 (H) |
| AST | 0 - 40 | U/L | 20.3 | 18 | 23.6 | 26.4 | 21.7 | 31.9 | 30.3 | 24.3 |
After completion of DCP treatment, the patient returned to the United States for cystoscopy follow-up at WVU Hospital. Cystoscopies performed on October 13, 2016, January 16, 2017, April 20, 2017, and on August 3, 2017 revealed no recurrence of tumor, and the resection area was observed to be clear of new growth on each occasion. Wash solutions collected during each cystoscopy were examined by a Cytologist and no malignant cells were observed.
Figure 2 shows the images taken during cystoscopy before resection (top panels) and after resection (2nd panels) on June 30, 2016. The residue of bladder tumor lesion observed during the second follow-up is shown in the 3rd panels, and during the third follow-up in the bottom panel. The resection site appeared to be healing.
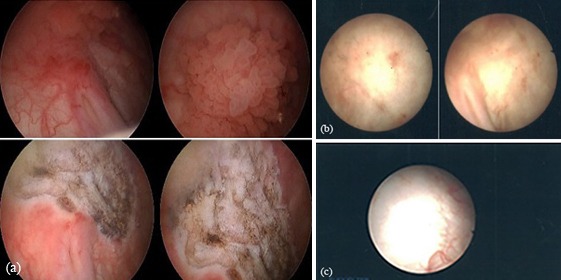
The DCP chemotherapy received by this patient – and follow-up observations of his resection site - may not prove DCP efficacy is superior. However, the patient has received DCP only and there is no evidence to date of tumor recurrence. Also of note, the patient experienced tolerable side effects during DCP chemotherapy.
Molecular mechanism studies discovered that dicycloplatin-induced DNA damage response in normal ovarian epithelial IOSE364 and ovarian cancer A2780 cells was investigated at the WVU Molecular Medicine Core Facility to determine the DCP-activated signaling pathway. After 1-hr drug exposure at IC50 doses (3.79 μM), several kinases of the DNA damage repair pathway were activated in cancer cells only. In ovarian cancer A2780 cells, the activation included phosphorylation of p53 at Ser15, phosphorylation of Chk2 at Thr68, and phosphorylation of BRCA1 at Ser1497, respectively. There were also increased proteins of p53 and p21. However, the phosphorylation of these kinases (p-p53, p-Chk2 and p-BRCA1) was not shown in normal ovarian epithelial IOSE364 cells, and the expressed proteins of Chk2, p53 and BRCA1 were not up-regulated by DCP in the normal cells. The p53 regulated downstream kinase p21 did not express due to p53-activation failure (data not shown). These data may explain the observations that the patient did not experience serious side effects during DCP treatment.
Worth noting, the results of WVU molecular mechanism studies and two in vitro investigations in China (data not shown) indicate that DCP mainly affects tumor cells, largely bypassing normal cells. Chinese researchers using Atomic Force Microscopy (AFM) observed that dicycloplatin does not affect single DNA molecules of normal cells, unlike other platinum drugs, such as oxaliplatin, which alters DNA structure of normal cells. In another investigation in China, DCP, at certain concentrations, mainly affected cancer cells (lung carcinoma and melanoma cells) but did not affect or only slightly affected normal cells (dermal fibroblast and mammary epithelia cells). These results are consistent with the medical data for - and the observations of - the first American cancer patient to receive DCP chemotherapy. The patient did not suffer from serious myelosuppression or other toxic effects.
In addition, symptoms and clinical data for the patient suggest important considerations for DCP chemotherapy - and possibly for other platinum drugs - relating to dose management and administration schedule. That is, it may be possible to treat cancer patients with mid-level doses (300 mg to 550 mg of DCP) instead of higher doses (650 mg or 800 mg of DCP), and more frequent IV infusions (weekly instead of bi-weekly or every three weeks), to reach target dosage and efficacy with fewer adverse effects.
Finally, the dicycloplatin experience of the patient - carefully documented in the United States and in China - is very much the same as reported by Chinese physicians, researchers and patients. Studies at WVU illuminate the underlying molecular mechanisms and gene activation process. This collaborative effort is an excellent example of an East-West coming together to better understand and improve platinum drug chemotherapy.
ConclusionTop
A platinum-based anticancer drug as effective as cisplatin and carboplatin – with bearable side effects – is an important therapeutic advance. Clinical investigation of DCP in the United States and Europe is warranted.
Acknowledgements
The authors would like to thank Michael D. Mueller for his special contributions to this research project and for editorial assistance. We thank Professor Xuqing Yang for his scientific guidance of dicycloplatin research in coordination with drug development.
Competing interests
The authors declare that they have no competing interests.
ReferencesTop
[1]The World Cancer Research Fund International. Cancer facts and figures. 2015.Article
[2]Cancer Research UK. Worldwide cancer statistics. 2015.Article
[3]The American Cancer Society. Cancer facts and figures. 2016.Article
[4]Yang XQ, Yu JJ, Guo Y, Mueller MD. Dicycloplatin: A next-generation platinum drug for cancer chemotherapy. In Atta-ur-Rahman (Ed.) Frontiers in Clinical Drug Research - Anti-Cancer Agents. 2017; 4:3–54.
[5]Yu JJ, Yang XQ, Song QH, Mueller MD, Remick SC. Dicycloplatin, a novel platinum analog in chemotherapy: Synthesis of Chinese preclinical and clinical profile and emerging mechanistic studies. Anticancer Research. 2014; 34:455–464.Article
[6]Yang XQ, Jin XL, Song QH, Tang KL, Yang ZY, et al. Structural studies of dicycloplatin: An antitumor supramolecule. Scientia Sinica Chimica. 2010; 53(6): 1346–1351.Article
[7]Liu KJ, Guan ZZ, Liang Y, Yang XQ, Peng J, et al. A double-blind, randomized phase II study of dicycloplatin plus paclitaxel versus carboplatin plus paclitaxel as first-line therapy for patients with advanced non-small-cell lung cancers. Arch Med Sci. 2014; 10(4):717–724.Article Pubmed
[8]Peng J, Wu HY, Guan ZZ. Chemotherapy-naïve patients with advanced non-small cell lung cancer–single institution experience. Chin J Clin Oncol. 2009; 12:711–714.
[9]Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380(9859):2095–2128.Article Pubmed
[10]University of Rochester Medical Center. Scientists find one reason why bladder cancer hits more men. 2007.Article
[11]Hemelt M, Zeegers MP. The effect of smoking on the male excess of bladder cancer: A meta-analysis and geographical analyses. Int J Cancer. 2000; 124(2):412–419.Article Pubmed
[12]Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: A meta-analysis of epidemiologic studies. Cancer. 2000; 89(3):630–639.Article Pubmed
[13]van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP. Quantified relations between exposure to tobacco smoking and bladder cancer risk: A meta-analysis of 89 observational studies. Int J Epidemiol. 2016; 45(3):857–870.Article Pubmed
[14]European Association of Urology (EAU) guidelines.Website
[15]Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999; 353(9165):1689–1694.Article Pubmed
[16]Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003; 349(9):859–866.Article Pubmed
[17]Kassouf W, Aprikian A, Black P, Kulkarni G, Izawa J, et al. Recommendations for the improvement of bladder cancer quality of care in Canada: A consensus document reviewed and endorsed by Bladder Cancer Canada (BCC), Canadian Urologic Oncology Group (CUOG), and Canadian Urological Association (CUA), December 2015. Can Urol Assoc J. 2016; 10(1-2):E46–E80.Article Pubmed
[18]Hall R. Updated results of a randomised controlled trial of neoadjuvant cisplatin (C), methotrexate (m) and vinblastin (V) chemotherapy for muscle-invasive bladder cancer. Proc Am Soc Clin Oncol. 2002; 21:178A.
[19]Sherif A, Holmberg L, Rintala E, Mestad O, Nilsson J, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: A combined analysis of two Nordic studies. Eur Urol. 2004; 45(3):297–303.Article Pubmed
[20]Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005; 48(2):202–205.Article Pubmed
[21]Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005; 66 (6 Suppl 1):4–34.Article Pubmed
[22]Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: A meta-analysis of the published results of randomized clinical trials. J Urol. 2005; 174(1):86–91.Article Pubmed
[23]Martínez-Piñeiro JA, Jiménez León J, Martínez-Piñeiro L Jr, Fiter L, Mosteiro JA, et al. Bacillus Calmette-Guerin versus doxorubicin versus thiotepa: A randomized prospective study in 202 patients with superficial bladder cancer. J Urol. 1990; 143(3):502–506.Article Pubmed


